Hello Everyone,
I waited till this week to post an update because I wanted to reflect on what was my last day with the Bio Base program this past Friday. As you know, I’ve been working with the Science Sisters program there helping to teach a small class of 3rd and 4th grade girls. This semester we were doing a Food Science program and we were talking about how and why salt is useful for making ice cream. I wanted to do two things this week: 1) explain the lesson and help you learn about ice cream and 2) tell you what I learned in this program.
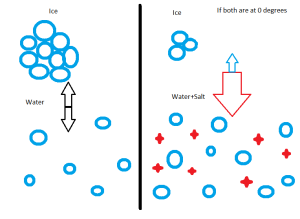
Firstly, ice cream! It’s getting to be summer time and it’s hot out so we’re all thinking about it. If you’ve made ice cream as a kid with your grandparents like me you might know that you need to make a bag of salt and ice to get the cream to freeze. When I asked some of my class why we would use ice and salt the most common answer I got was: it helps the ice melt. While this is true I made sure to ask them a follow up: so if all we wanted was melted ice why not just use water? It goes into the thermodynamic principals behind mixing; something we’ve talked about with our molecular yoga. What we are creating is a mixture of ice and water. Ice and water are in equilibrium at 0 degrees Celsius. One **very** important thing to remember about equilibrium is that it does not mean that all of the water is becoming ice. There is another factor related to Free Energy, which we talked about before, called Chemical Potential. The Chemical Potential is essentially the ability for a chemical to undergo a reaction, in this case to go from a liquid to a solid. At 0 degrees the total chemical potential is zero and the Free Energy is at a minimum. So if I have a box of ice and water at 0 degrees Celsius in which no heat can leave (called “adiabatic”), I can expect that, on average, for every water molecule that melts into a liquid, one will freeze into a solid.
Now what happens when we add a salt to this mixture? The salt particles try to mix in with the solid form of water, the ice. They prevent the ice from packing together nicely and they add an extra component to the chemical potential. From this we can say that in order to reach the new equilibrium we would have to set our mixture to a lower temperature. Therefore we can end up with a solution with liquid water that is actually colder than 0 degrees Celsius. You can try this experiment yourself. Take two glasses and fill them up with equal amounts of water. Add a bunch of salt to one of them and then add equal amounts of ice to each. Wait a little while and then dip your fingers into each of them or poor them out. You’ll notice that the mixture with salt feels much much colder. Also what you can realize is that you need a liquid below zero degrees in order to freeze the ice cream because just like salt water, the ice cream is not pure water but is, again, a mixture.
This experiment and lesson was really fun. The group had a great time with this experiment and we ended up with some fairly tasty ice cream too! I had a great time teaching this class and it helped me to reevaluate why I wanted to teach and why I wanted to be a part of outreach programs. I said before back in November that an important reason to be a part of scientific outreach programs was to help create a better informed public. I still feel very strongly about this reasoning but one thing that stuck with me as I was doing this was a question a friend posed to me: why teach a class of girls specifically? The true answer was that I didn’t know the BioBase was specifically for girls or a part of the Girls Club when I started. Now, however, I do think that it’s important to reach out to young girls in particular. It’s not just that we live in a world where we don’t encourage women enough to participate in science enough. We live in a world where we actively discourage women from going into science and young women in particular. We live in a world where there are forces who would kidnap and imprison girls for trying to get an education. We live in a world where women who do try to pursue a secondary education have to fear for their safety on their college campuses. We live in a world where even if a woman is lucky enough to make it to having a professorship position she can be as low as half as likely as a man to get tenure. And people look around and ask where all of the female professors are. This is why I want to continue science outreach programs for girls and young women. To combat the discouragement and to give at least some support from an early age.
Thank you for reading this week, I hope I’ve given you both some cold and hot things to think about. Please feel free to discuss!
Sincerely,
GRW